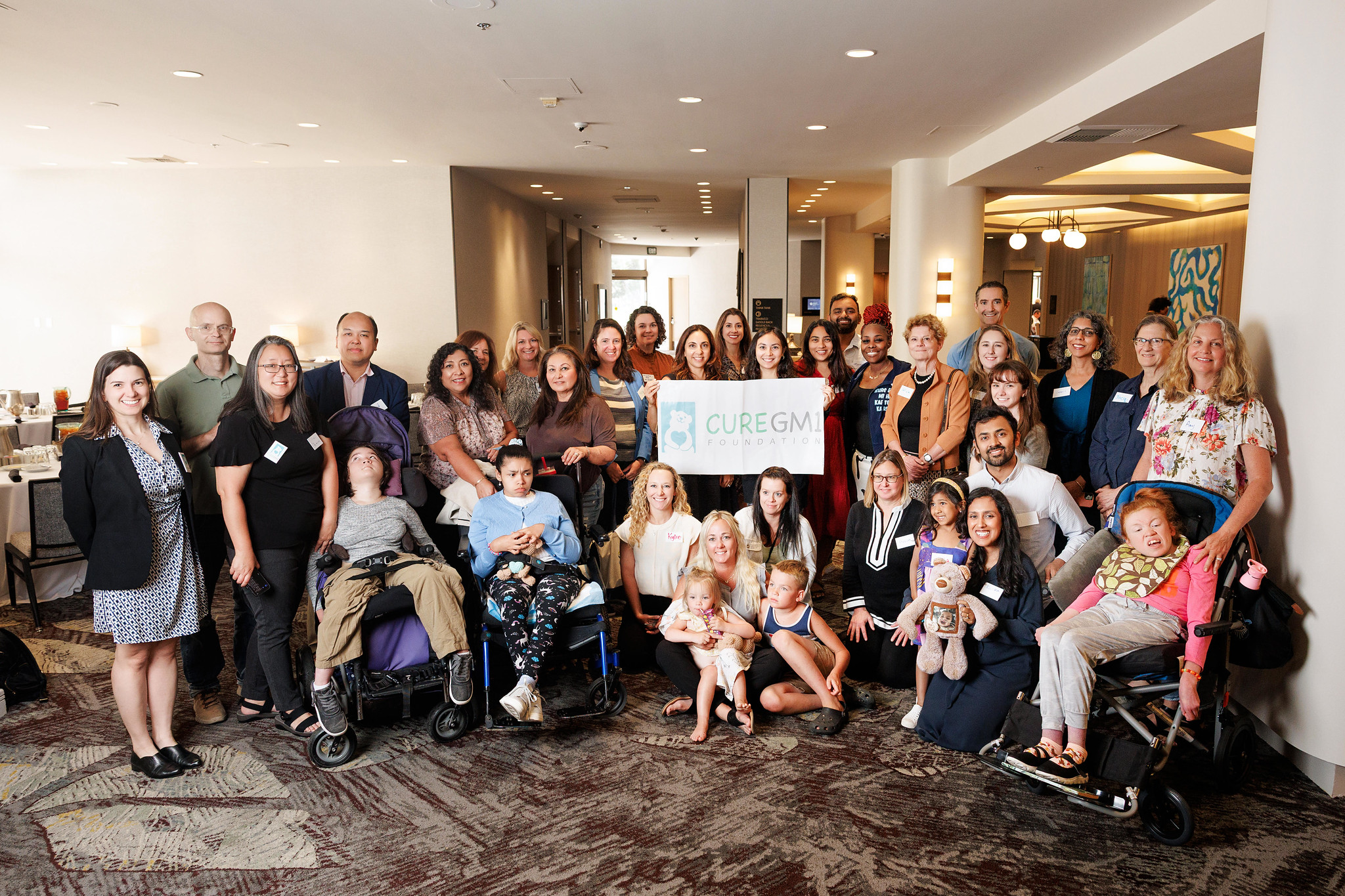Our Trusted Partners & Awards
WHAT WE DO
We are changing the Status of GM1
GM1 gangliosidosis was first identified as a distinct disease in 1968 and yet, patient advocacy and a strong focus on GM1 was sorely lacking until recently. Cure GM1’s focus is always on GM1. The Cure GM1 Foundation’s mission is to fund research and to support patient advocacy and drug development for the benefit of all those who suffer from GM1. This 501(c)(3) nonprofit organization was founded by parents of children who suffer from GM1. The U.S. federal government approved the formation of our organization in April of 2015.

$6M+
Cure GM1 has raised significant funding and driven meaningful progress, but more funding is still required.
5
Since Cure GM1 was founded, there have been 5 clinical trials, whereas prior to Cure GM1’s existence, there were none.
95%
95% of the 10,000+ rare diseases have no treatments.
Community Stories
Take action and join us
Participate and Take Action
Join the GM1 Census and Patient Registry
GM1 families have the power to change the understanding of GM1. The GM1 census is a critical tool to deepen the understanding of GM1, mapping the diagnostic journey, identifying common symptoms and demonstrating to biotech companies and interested parties that our community is research-ready. Join now.

Annual International GM1 Community Conference
Our Fall annual conference is an extremely valuable resource to GM1 families and to researchers and biotechs working on GM1. The presentations provide incredibly valuable information regarding GM1, clinical trials, caregiving tips and more.

Do It for GM1
Do It For GM1 is a movement to bring us closer to a world where children with GM1 can look forward to longer, healthier lives.
Whether you’re an athlete, a baker, a swimmer, or someone who simply wants to make a difference, there’s a way for you to contribute. Choose the activity that speaks to you, rally your community, and help us accelerate critical research and support families affected by GM1 gangliosidosis.
Rare Disease Day
Raising awareness and generating change for the 300 million people worldwide living with a rare disease, their families and caregivers.
International GM1 Gangliosidosis Awareness Day
Organized by Cure GM1 in collaboration with multiple patient advocacy groups, International GM1 Gangliosidosis Awareness Day is a worldwide movement. Increased advocacy and the funding of research and drug development can help advance the creation of possible treatments and ultimately improve and save lives.
Take Meaningful Action and Help Advance our Mission
Our work depends on volunteers and active participation in GM1 research, advocacy, and fundraising. Watch a video about our critically important work.
Helpful resources
Help Build Resources, Tools, knowledge and Community
Donations, volunteers, and our global community fuel our critically important work to help develop and advance possible treatments.
GM1 and rare disease community
collaboration, advocacy and community are critical
Cure GM1 has been instrumental in helping build community and to structure meaningful projects and collaboration amongst stakeholders. Some highlights include the organization of a natural history data sharing effort, the development of a newborn screening assay, and interactions with the U.S. Food and Drug Adminstration, FDA.
Jenny Bragg,
GM1 parent
“If I could change Clara’s future, I would. Nevertheless, I am forever grateful for the impact she has had on my life as well as countless others.”
Kylie Harrison,
Board Member
“People are telling us there’s nothing we can do for our daughters, we’re not accepting that. There is something we can do and that is to raise awareness and raise funding for them and for others.
Christine Waggoner
Founder
“Cure GM1 is laser-focused on creating a legacy of hope and change by navigating the many challenges of ultra-rare disease drug development and research.”
Emil Kakkis, Advisor
“I believe that GM1 is at a place where it can get treated. But you have to be vigilant and fight for support to ensure that the right things get done and that treatments become available.”
SAFE + EASY DONATIONS and fundraising
Ways to Donate and Fundraise
Download our fundraising manual. Read our refund policy.

Contact us for help
Email info@curegm1.org
We’re Changing
GM1 Research Globally
Our work and advocacy contributes to brings increased awareness, scientific knowledge, and financial investment.
36+
PARTICIPANTS IN TRIALS
5
CLINICAL TRIALS TO DATE
9
SCIENTIFIC PUBLICATIONS
2
PUBLICLY AVAILABLE MOUSE MODELS