
January News
Thank you for an amazing year in 2024!
The impact of your support gives hope to children, brings comfort to families, and accelerates the research that could change lives. Your support means that not a single moment will be lost in our pursuit of better treatments and support for families affected by GM1.
Thank you for choosing to stand with us. Your decision to support the Cure GM1 Foundation makes a real difference in our community. We look forward to sharing our progress and achievements with you throughout 2025. We are truly grateful for your support!
Read on to celebrate our 10th anniversary year by exploring our 2024 Impact Report, meeting inspiring youth volunteers, supporting groundbreaking research, and joining the fight against GM1 gangliosidosis through events, advocacy, and new biotech breakthroughs in 2025!
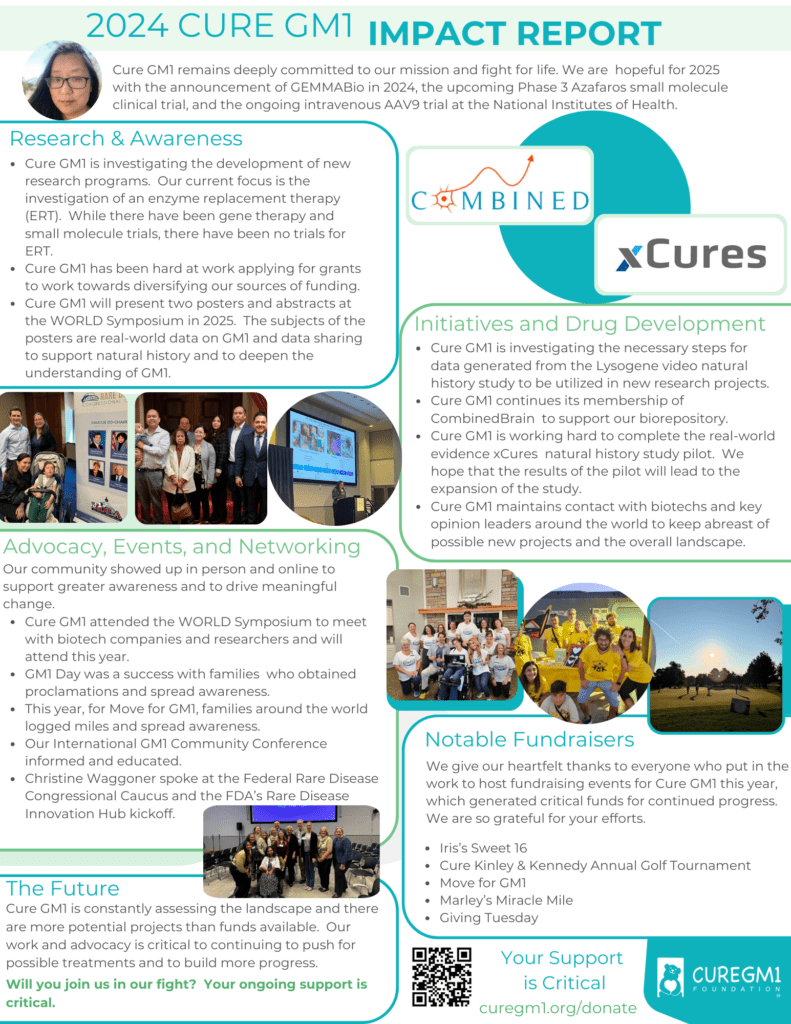
2024 Cure GM1 Impact Report
COMMUNITY ENGAGEMENT
Our 2024 Impact Summary is now available! While this year brought challenges, we remain steadfast in building hope and taking action to shape a brighter future for GM1.
Cure GM1 is proud to share our most significant accomplishments of 2024. Several projects and initiatives gained momentum and came to fruition this year, marking important progress in our mission. The work we do together is transforming the GM1 landscape and will continue to make an impact for years to come.
This progress would not be possible without the incredible support of our donors and the GM1 community. Thank you for being an essential part of this journey!

Celebrates 10 year Anniversary
10 Year Anniversary
The U.S. Federal government approved Cure GM1 Foundation on April 15th, 2015 as the only 501(c)(3) nonprofit organization in the U.S. entirely dedicated to GM1 gangliosidosis research, drug development, advocacy and awareness.
During the time since Cure GM1 was first founded:
- There were 4 clinical trials whereas prior to the existence of Cure GM1, there were none.
- Dozens of children received chances in clinical trials.
- We’ve built a community of thousands of people in support of an ultra-rare disease.
- New clinical trials are upcoming and patient advocacy groups are key in attracting investors and biotechs to rare diseases.
Advocacy and progress in rare diseases require consistency, grit, and determination. GM1 gangliosidosis is a terrible devastating condition and the dire need of our community for help requires a long-term commitment and ongoing advocacy.

Will You Join Us in Making A Difference
10 Year Anniversary
Will you join us in making a difference and an ongoing commitment? Whether honoring the memory of a loved one, fighting for treatment, a professional interest, or simply general support, your kindness and generosity is truly needed.
Recurring donations in 2025 can make a big difference. Will you commit to a multiple of $10 per month in honor of our 10 year anniversary? Did you know that on average a rare disease treatment takes 15 years to reach approval? This is far too long! Worse, due to lack of funding, many programs are halted and shelved, even when the treatments show promise. We can’t let this continue to be our reality. Long-term commitment is required to make a lasting impact
You can help spread the word and show your solidarity by downloading and applying our specially designed Anniversary Frame for your Facebook profile picture.

A New Year and Renewed Commitment To Cure GM1
COMMUNITY ENGAGEMENT
Imagine if there had been consistent fundraising since GM1 gangliosidosis was first distinguished as its own condition in 1968. We are making up for lost time. Will you make a New Year’s Resolution to join our fight for life? In 2025, Make a Plan to Participate and Join us!
Host Your Own Event
Whether it’s for Rare Disease Day, GM1 Day, Move for GM1, or something entirely unique, your creativity can fuel change
Mark Your Calendar for 2025 Key Events
- Rare Affair at the JPM Healthcare Conference in San Francisco – Jan. 12th
- World Symposium in San Diego – Feb. 3-7th
- Rare Disease Day – Feb. 28th
- GM1 Day – May 23rd
- Cure GM1’s Annual Conference – Free for families – Late Summer/Fall
- Move for GM1 – Late Summer/Fall
Make a Plan for Rare Disease Day 2025
COMMUNITY ENGAGEMENT
Rare Disease Day is an annual global event dedicated to raising awareness and advocating for those living with rare conditions, like GM1 gangliosidosis. It’s a day to come together as a community, amplify our voices, and make a lasting impact.

Rare Disease Day
Support in February by having the Facebook Picture Frame>
Raising awareness and generating change for the 300 million people worldwide living with a rare disease, their families, and carers. See the Cure GM1 Rare Disease page. Also, learn more at the Rare Disease Day Website>

Rare Disease Day Photo Contest
Submit your photo and share how you’re making a difference. Enter our Rare Disease Day photo contest>

Registration for Rare Disease Week on Capitol Hill
Save the date for Rare Disease Week on Capitol Hill, February 24-26, 2025! #RareDC2025. Read More>

FDA-NIH Rare Disease Day
The 2025 FDA-NIH Rare Disease Day is a two-day meeting on Thursday, February 27 and Friday, February 28, 2025, from 10 a.m. to 4 p.m. EST each day. The meeting will take place online (virtually) and in-person in Bethesda, Maryland, on the NIH campus in the Natcher Conference Center. For more information about NIH campus security and how to get to NIH, please visit FDA-NIH Rare Disease Day

Meet Ben
Community Engagement
Diagnosed with juvenile GM1 gangliosidosis just days before Christmas 2023 at age 9, he’s the sweetest, most mischievous 10-year-old you’ll ever meet. Once learning to read, ride a bike, and write words, his world began to change—clumsiness turned to setbacks, and by age 7, Ben could no longer read the short books he once loved.
Ben’s diagnosis journey was anything but straightforward. From countless tests to relentless advocacy by his mom, answers came with heartbreak. GM1 changed their family forever, but they’ve found hope, resilience, and a supportive GM1 community along the way.
Read their full story and discover why supporting rare disease research, like the work of the Cure GM1 Foundation, matters more than ever.

Attend the 21st Annual WORLD Symposium
COMMUNITY ENGAGEMENT
Free registration for families impacted by lysosomal storage diseases. Want to meet up? Cure GM1 will be attending to connect directly with physicians, researchers, biotech companies, and key stakeholders.
Cure GM1 submitted two abstracts and these abstracts were accepted for poster presentations. The work leading up to these submissions and posters took place over a period of years. By presenting abstracts and posters, we can draw attention to GM1 gangliosidosis, present our projects and work to the broader scientific community, and build broader support for GM1 research and drug development.

Genotypic Heterogeneity in GM1
COMMUNITY ENGAGEMENT
Authors: Caroline Hastings1, Ashley Cogell2, Vanessa Rangel Miller3, Christine Waggoner
Affiliations:
1. UCSF Benioff Children’s Hospital Oakland, Oakland, CA
2. PicnicHealth and AllStripes Research, San Francisco, CA
3. Cure GM1 Foundation, Albany, CA

The GM1 Natural History Data Sharing Collaborative: A roadmap for accelerating rare disease research
COMMUNITY ENGAGEMENT
Authors: Christine Waggoner 1 , Kathleen Kirby 1 , Vanessa Rangel Miller 1 , Roberto Giugliani 2 , Jeanine Jarnes 3 , Cara Weismann 4 , David Whiteman 1 , James Wilson 5 , Rafael Escandon 1 , Collin Hovinga 6
Affiliations:
1. Cure GM1 Foundation, Albany, CA
2. Casa dos Raros, Porto Alegre, Brazil
3. University of Minnesota, Minneapolis, MN
4. Orphan Disease Center, University of Pennsylvania, Philadelphia, PA
5. Institute for Life Changing Medicines, Philadelphia, PA
6. Critical Path Institute

EveryLife Scientific Workshop
COMMUNITY & ENGAGEMENT
In 2024, Christine attended an important workshop on therapy development for small populations and ultra-rare diseases hosted by the EveryLife Foundation. Curious about the insights? Explore the highlights in the summary: Therapy Development for Small Populations: Evidence, Implications, & Policy in Characterizing Ultra-Rare.
Download Scientific Workshop Information>
Welcoming our New Youth Volunteers to
the Cure GM1 Team
COMMUNITY & ENGAGEMENT
Cure GM1 is pleased to announce three new volunteers who are volunteering this year to deepen their understanding of GM1 and rare diseases. Two of our volunteers are siblings to loved ones impacted by GM1.
Make a resolution to volunteer for Cure GM1 in 2025. Our work relies on the participation of the community. Please let us know if you would like to help this year!

Carter Dooley
VOlunteer
Carter Dooley, Iris’s brother, creating a 3D computer graphics video to demonstrate intracerebroventricular enzyme replacement therapy, injection inserting beta-galactosidase made absent by the disease back into the cerebrospinal fluid.
“I think people should support rare disease foundations to prevent the torture a person with a rare disease endures. I think they should imagine themselves put into the shoes of a person with a rare disease.”
-Carter

Jaden de Venecia
VOlunteer
Jaden de Venecia, Kate’s brother, creating informational posts for Rare Disease Day. These posts are designed to raise awareness about rare diseases by sharing important facts and highlighting their impacts.
“Helping others is not just about changing their lives—it enriches my own. It reminds me of the power of connection and the importance of giving back.”
-Jaden

Kian De Souza
VOlunteer
Kian De Souza’s projects are to make a GoFundMe for Cure GM1 to raise funds for research and drug development and to build a pitch deck for potential investors.
“Volunteering for the Cure GM1 Foundation is important because it raises awareness on GM1 and allows me to help people in my community.
-Kian

Priority Review Voucher (PRV) Not Renewed by Congress
ADVOCACY
More advocacy is required for this to be passed in 2025! We must act to ensure this incredibly important incentive is renewed! Additional advocacy will be necessary to reinstate the PRV. This incentive is a useful tool to attract investment into all rare diseases and does not cost the government any money.
Read More on Facebook>
Read a heartfelt message from a rare disease father highlighting the critical role of the PRV (Priority Review Voucher) and the importance of policies that support rare disease drug development. Read More on LinkedIn>

Opinion: What Accelerated Approval’s Naysayers Miss
ADVOCACY
“By speeding lifesaving drugs’ way to market and focusing on the underlying causes of disease, the pathway has helped save many lives.
Patients with rare diseases are in dire need of effective treatments. Yet some academics are pushing regulators to deny them access to medicines that could save their lives.” Read More>
Thank you to all those who contributed to our Giving Tuesday and Year-End Fundraiser!
FUNDRAISING
Thank you to everyone who supported our Giving Tuesday and Year-End Fundraiser! A heartfelt thanks to three bereaved families who honored their loved ones by contributing to a legacy of hope.

Fiona’s Kindness Project Eggroll Fundraiser
FUNDRAISING
Fiona’s family turned their love for her into action, raising funds through their heartwarming eggroll fundraiser to honor her memory.

Harrison’s Heroes Grinch Tree Fundraiser
FUNDRAISING
Harrison’s family brought holiday cheer and hope through their creative Grinch tree fundraiser, supporting a meaningful cause in his name.

Niam’s Memorial Fundraiser
FUNDRAISING
Niam’s family honored their loved one with a touching memorial fundraiser, creating a legacy of compassion and support.

See’s Candies
FUNDRAISING
Thank you for supporting the Valentine’s 2025! We’ll receive profits from any items you purchase below. Order more to help us reach our fundraising goal!
Valentine’s Day: Virtual Card Request
FUNDRAISING
Spread the love this Valentine’s Day! Personalize your card, and we’ll deliver it straight to your loved one’s inbox. Let’s make their day extra special!

Buy a bouquet and support Cure GM1!
FUNDRAISING
With Flowers & Fundraising, “Just picked” flowers are shipped directly from growers to you and benefit our organization. Also you can use RaiseRight.com with 1-800-flowers

Purchase Prints and Cards to Support Cure GM1 year-round with Minted.com!
fundraising
Use the code FUNDRAISECGM1 at checkout to receive a special discount on your purchases. Plus, 15% of your purchase will be donated to charity. It’s a simple way to give back while shopping for beautiful, high-quality products. Every purchase helps make a difference for those affected by GM1 gangliosidosis.
Visit Minted.Com to support Cure GM1

GemmaBio Secures $34M to Advance Gene Therapies for Rare Diseases
BIOTECH NEWS
Jim Wilson’s GemmaBio has raised an additional $34M to help bring gene therapies for rare diseases closer to reality. Learn more about their efforts and what this means for rare disease treatment: Read the full story

Advocating for Transparency in Gene Therapy
BIOTECH NEWS
Board Member Rafael Escandon and colleagues address the need for greater transparency and collaboration in gene therapy trials, particularly regarding patient safety and AAV-related deaths. Read his insightful article to learn more: Full article here

Idorsia Publishes RETRIEVE Study on Early-Onset Lysosomal Storage Disorders
BIOTECH NEWS
Idorsia has published a significant natural history study on pediatric patients with early-onset GM1 gangliosidosis, GM2 gangliosidoses, and Gaucher disease type 2. Learn more about their findings in the RETRIEVE study, released on December 4th: Read the study here.


