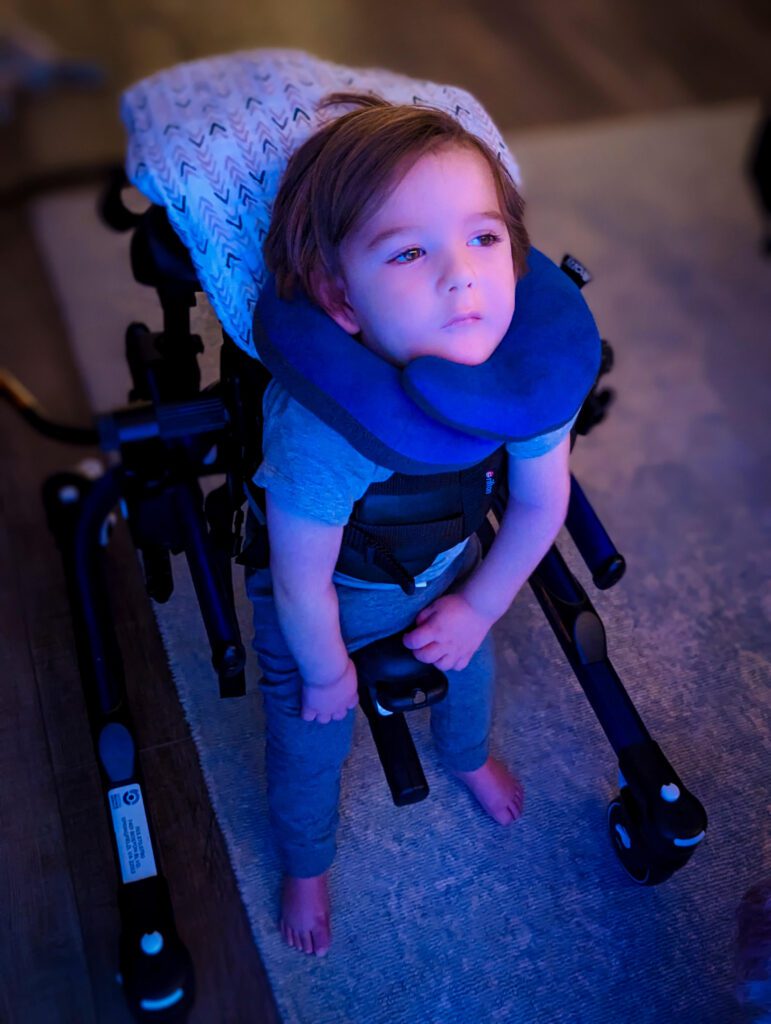
Aidan’s story, by his mother
“This is my son, Aidan, who was diagnosed with GM1 gangliosidosis at just 15 months old.”

How has GM1 gangliosidosis affected your child?
My son is now 3 and a half and in the time since his diagnosis, he has gone from a child who could walk, talk, giggle, play, climb furniture, and devour a turkey leg faster than most adults I know to now being unable to move his limbs, hold his own head up, cry, or even smile.

How does GM1 impact your family?
Over the last two years I have used social media to raise awareness for GM1 and have connected with dozens of families across the world whose children have also been diagnosed with GM1 or other very similar fatal pediatric conditions.
One of the first questions they always ask is, “Do you know of any treatments?” And time after time I have to repeat the same devastating words, “No. There are currently no approved treatments for GM1, and almost every clinical trial for a treatment has been halted in the last few years due to lack of funding and concerns about the ability to recoup R&D costs due to it being a rare disease with a small population.”
What do you wish people understood
more about rare diseases?
70% of rare diseases are exclusively pediatric onset and overall, 95% of them have no approved treatments. Furthermore, it takes 15 years on average for a treatment to be developed and approved by the FDA, 3 times longer than my own son’s current life expectancy and the life expectancies of many other children with similar rare diseases. It is imperative that there be legislation in place to:
- Increase financial support for rare disease drug development
- Reduce the time it takes for rare disease treatments to be approved
- Incentivize companies to develop treatments for rare disease populations and for diseases such as GM1 gangliosidosis.
Why should people support the Cure GM1 Foundation and rare diseases?
Without such legislation and the financial support for rare disease research and grants, our children will continue to suffer everyday while we, as their parents, must watch them slowly fade until one day we must bury them. Your support for our advocacy would be a step towards a future where families like mine aren’t given the most devastating news of our lives and then left to go home with no hope of treatment options within our children’s lifetimes.
As of the publication of this post, there are several key areas of focus in rare disease patient advocacy:
- The renewal and continued support of the Priority Review Voucher to encourage investment into rare disease programs. To read more, see the FDA website.
- Leveraging the accelerated approval pathway and biomarkers to a greater extent for rare diseases. To learn more, see this page from the EveryLife Foundation.
Sweet Aidan passed away just two weeks before his 4th birthday on February 9th, 2025.