

Goodbye March & Hello April.
The CURE Gm1 Catalyst
Dive into Cure GM1 Catalyst April’s edition filled with heartwarming welcomes, crucial updates, and inspiring opportunities to engage and make a difference. From welcoming new faces to our global community, gearing up for GM1 Day, to supporting legislative advocacy, partnering with biotechs, and paving the way for GM1 research, we’re moving forward together.
Plus, discover how you can contribute to our cause through various fundraising efforts and events. There’s something for everyone in this journey toward hope and healing.
COMMUNITY ENGAGEMENT
INTERNATIONAL GM1 AWARENESS DAY
FUNDRAISING
LEGISLATION
BIOTECH NEWS
APRIL ANGELVERSARY & BIRTHDAYS
RaiseRight Webinar
COMMUNITY ENGAGEMENT
Join our upcoming webinar, Tuesday April 16th to discover how your everyday purchases can effortlessly contribute to Cure GM1. Learn the simplicity of using gift cards from over 750 major retailers to support our cause. Imagine turning your grocery shopping, gas fill-ups, and coffee runs into significant donations. Register now to find out how every swipe can bring us closer to a cure. Can’t join, see our RaiseRight instructions by clicking the Learn More.


2024 Virtual Annual Conference
COMMUNITY ENGAGEMENT
Please save the date and prepare to join us on September 20, 2024, the conference will be held virtually. This significant event is your gateway to the latest in research, trials, and more.
Use the link below to suggest topics for the conference – your input is invaluable to us! Interested in being a sponsor to support the event? Always feel free to email us at info@curegm1.org.

David Weinstein, MD, MMSc
Read More
Say Hello
COMMUNITY ENGAGEMENT
Say hello to the newest members of our Cure GM1 community! We’re thrilled to welcome two incredible individuals who bring their unique talents and unwavering dedication to our mission. Each of them is passionate about making a tangible impact and is ready to contribute to our cause. Join us in warmly welcoming them into our community.

Anita Hernandez
Read More

A Proclamation for Violet & the GM1 Community
INTERNATIONAL GM1 AWARENESS DAY
On Rare Disease Day in Sacramento, an incredible moment of advocacy and awareness unfolded in honor of Violet and all those affected by GM1 Gangliosidosis. This heartfelt proclamation showcases the power of unity and the relentless spirit of our community. Let’s come together to celebrate this monumental achievement and continue our united fight for a cure. Watch Here. “The Law family are nothing short of heroes, honoring their beautiful daughter and helping other families in this cause,” state Sen. Steve Padilla said. Read More.
Need assistance submitting a proclamation in your state? Contact Anita Hernandez at anita@curegm1.org for all the resources and support you need.

Votes are In
INTERNATIONAL GM1 AWARENESS DAY
GM1 Awareness Day is fast approaching, and thanks to your votes, we’ve selected two top designs for our GM1 Day swag! Make sure to place your orders in time to showcase your support by May, 23, 2024
New to GM1 Awareness Day? No problem! We’ve got all the details you need to get involved and make an impact. Learn More.
Cure GM1 Surpasses $5M in funds raised!
FUNDRAISING
Years ago, we had not a single cent dedicated to our cause. Thanks to the incredible support of our global GM1 community, we can now plan for new research and have funds to be able to seed meaningful research and to attract greater investment. Cure GM1 has several projects in the contracting phase which will be announced as soon as possible. While there is still much work to do and funds are continually a challenge due to the high price of scientific research and drug development, we continue to push forward.
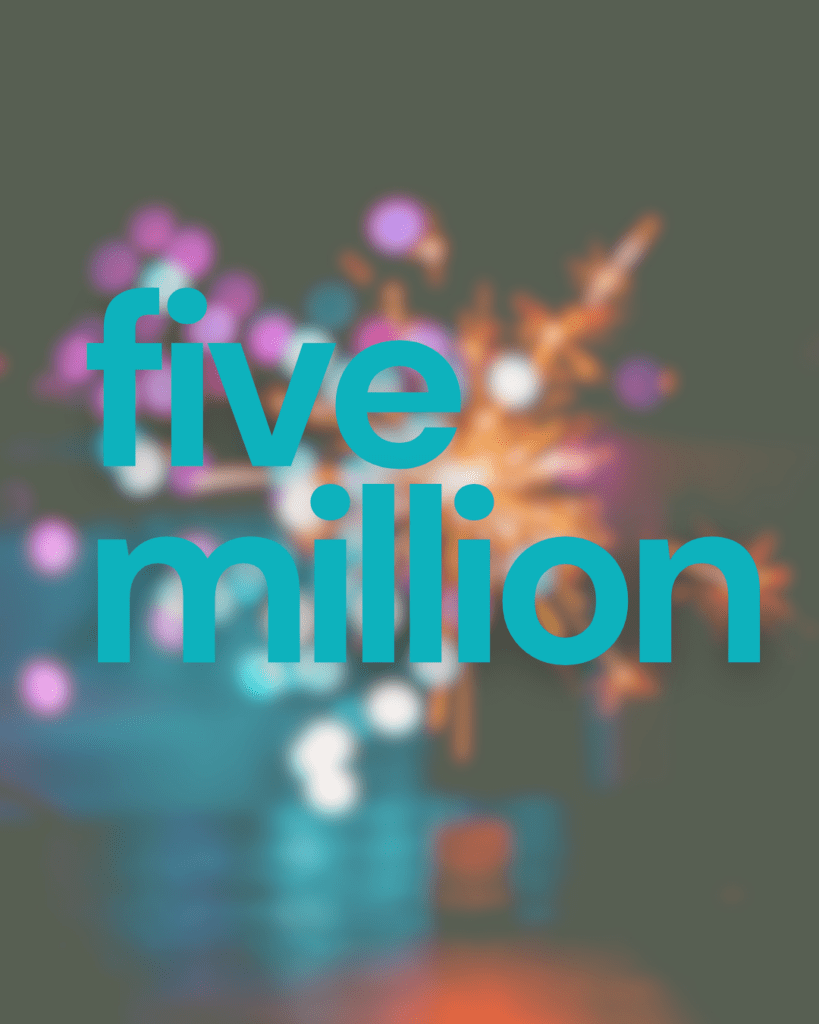
Spring Yumraising & Chocolates
FUNDRAISING
Support Our Spring 2024 Goal! Profits from your purchases below directly contribute to our cause. Every order brings us closer to our fundraising target!
Plan a Spring Fundraiser
FUNDRAISING
Spring into action with a fundraiser! Check our manual or share your new ideas with us at info@gm1cure.org
Flower Power Fundraising
FUNDRAISING
Brighten Mom’s Day with Beautiful Flowers! Orders ship starting April 1st, with the sale wrapping up on May 15th.

Creating Hope Reauthorization Act Introduced
Legislation
On February 15, the Creating Hope Reauthorization Act was introduced in the House by Representatives McCaul, Bilirakis, Burgess, Barragan, Eshoo, and Trahan. This legislation would reauthorize the Food and Drug Administration (FDA)’s Rare Pediatric Priority Review Voucher program, which incentivizes pharmaceutical companies to develop new drugs for rare pediatric diseases. To learn more on the Creating Hope Reauthorization Act, Click Here. To learn more about the PRV program, Click Here.

MLD Gene Therapy Approval
Biotech News
Can MLD serve as an example for GM1? Published research supports that the very same lentiviral gene therapy approach could be applied to GM1. What is lacking is an actual clinical trial and sponsor. This gene therapy approval for MLD is a hopeful example for lysosomal diseases, leukodystrophies and rare diseases overall. However, the reality is that this approval in the US and the overall process was lengthy with the first patients being treated 12 years ago. And this therapy was approved in Europe earlier. Advocacy is critical in continuing to push for progress in all rare diseases and for GM1. Read More.
FDA Approves First Gene Therapy for Children with Metachromatic Leukodystrophy. “In March the U.S. Food and Drug Administration approved Lenmeldy, the first FDA-approved gene therapy indicated for the treatment of children with pre-symptomatic late infantile, pre-symptomatic early juvenile or early symptomatic early juvenile metachromatic leukodystrophy (MLD).” Read More.

Azafaros completes Phase 2 trial in GM2 and NPC. Phase 3 to include GM1, GM2, and NPC Phase 3
Biotech News

RAINBOW Clinical Trial
Biotech News
Roberto Giugliani, MD, PhD, discusses the RAINBOW clinical trial testing the efficacy and safety of nizubaglustat for Niemann-Pick type C and GM2 gangliosidosis. Learn More.

Mother’s Day Around The Corner
With Mother’s Day fast approaching, it’s the perfect time to honor the remarkable moms in our Cure GM1 community. We’d love to shine some spot light & recognition to our Super Moms out there, so if you have someone in mind we would be thrilled to hear from you. Please reach out to anita@curegm1.org to share their story. Let’s celebrate these incredible women together!

Ways to Give – Your Support Matters
RaiseRight | Charity Miles | Facebook Fundraisers | Donate
Visit our Take Action page for more ways to support our community.
