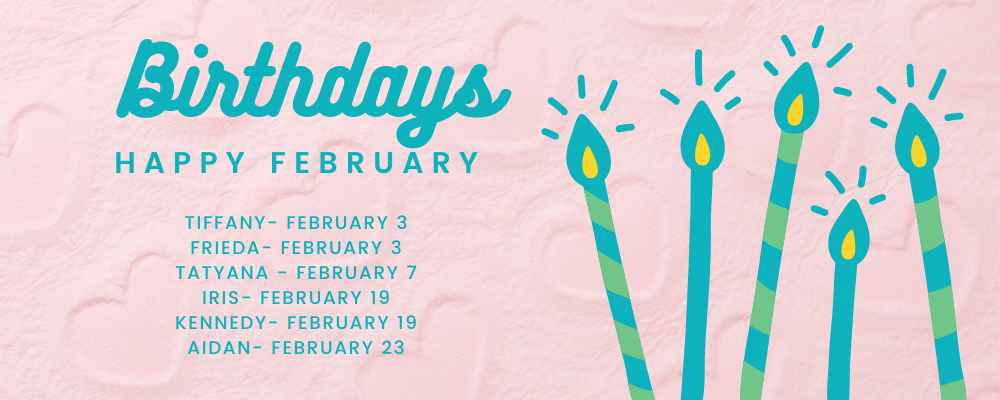
Cure GM1 Catalyst
This month is all about love, awareness, and action as we celebrate Valentine’s Day and Rare Disease Month. We are also celebrating our founder Christine’s daughter’s 17th birthday. For all families with children impacted by GM1, each birthday is a celebration, but the passage of time and the progression of the disease marks these milestones.
Learn more on how you can help us raise awareness by supporting GM1 Day proclamations, sharing Rare Disease Day campaigns, and exploring new GM1 swag in the GM1 Store.
We’ve also allocated $400,000 to advance enzyme replacement therapy and will be showcasing GM1 research at the WORLD Symposium. Stay engaged with the latest biotech news, advocacy efforts, and upcoming events.
COMMUNITY ENGAGEMENT
ADVOCACY
BIOTECH NEWS
RARE DISEASE DAY
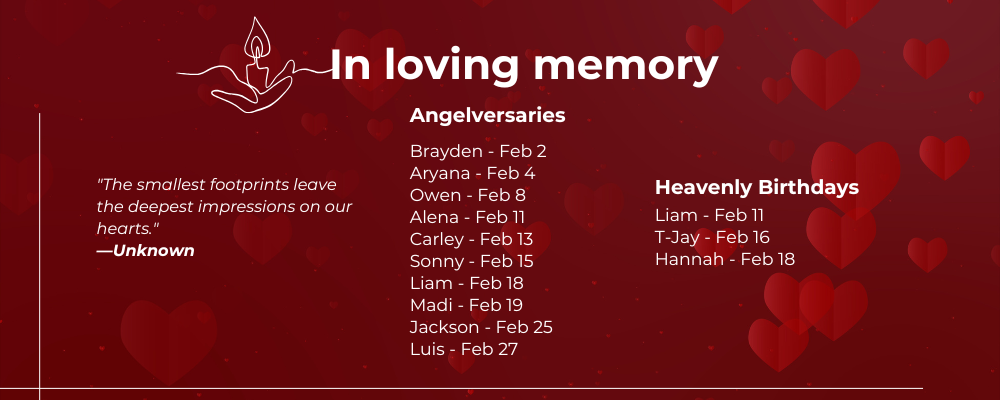
ANGELVERSARY & BIRTHDAYS
Make a Plan for Rare Disease Day 2025
RARE DISEASE DAY
Rare Disease Day is an annual global event dedicated to raising awareness and advocating for those living with rare conditions, like GM1 gangliosidosis. It’s a day to come together as a community, amplify our voices, and make a lasting impact.

Registration for Rare Disease Week on Capitol Hill
The 2025 Rare Disease Week is a multi-day event in Washington DC, hosted by RDLA (Rare Disease Legislative Advocates), and brings together rare disease advocates from across the country to make their voices heard by their Members of Congress. Participants are educated on policy proposals impacting the rare disease community and provided opportunities to advocate for policy changes directly to their Members of Congress. If you are interested in learning more, visit the Rare Disease Week website>

Rare Disease Day
Support in February by having the Facebook Picture Frame>
Raising awareness and generating change for the 300 million people worldwide living with a rare disease, their families, and carers. See the Cure GM1 Rare Disease page. Also, learn more at the Rare Disease Day Website>
Submit your photo and share how you’re making a difference. Enter our Rare Disease Day photo contest>

FDA-NIH Rare Disease Day
The 2025 FDA-NIH Rare Disease Day is a two-day meeting on Thursday, February 27 and Friday, February 28, 2025, from 10 a.m. to 4 p.m. EST each day. The meeting will take place online (virtually) and in-person in Bethesda, Maryland, on the NIH campus in the Natcher Conference Center. For more information about NIH campus security and how to get to NIH, please visit FDA-NIH Rare Disease Day
Every face tells a story. Every quote carries a fight

“Niam brought so much light, warmth, and pure unconditional love to our lives. In a world that’s always pushing us to chase the next milestone, Niam’s journey with GM1 forced us to slow down, live in the present, and at least try to let go of what we couldn’t control. I’ll always treasure the little moments—those quiet, simple moments we often take for granted. Niam showed us that a life isn’t about how long it lasts but how much love it holds—and his love will stay with us forever.”
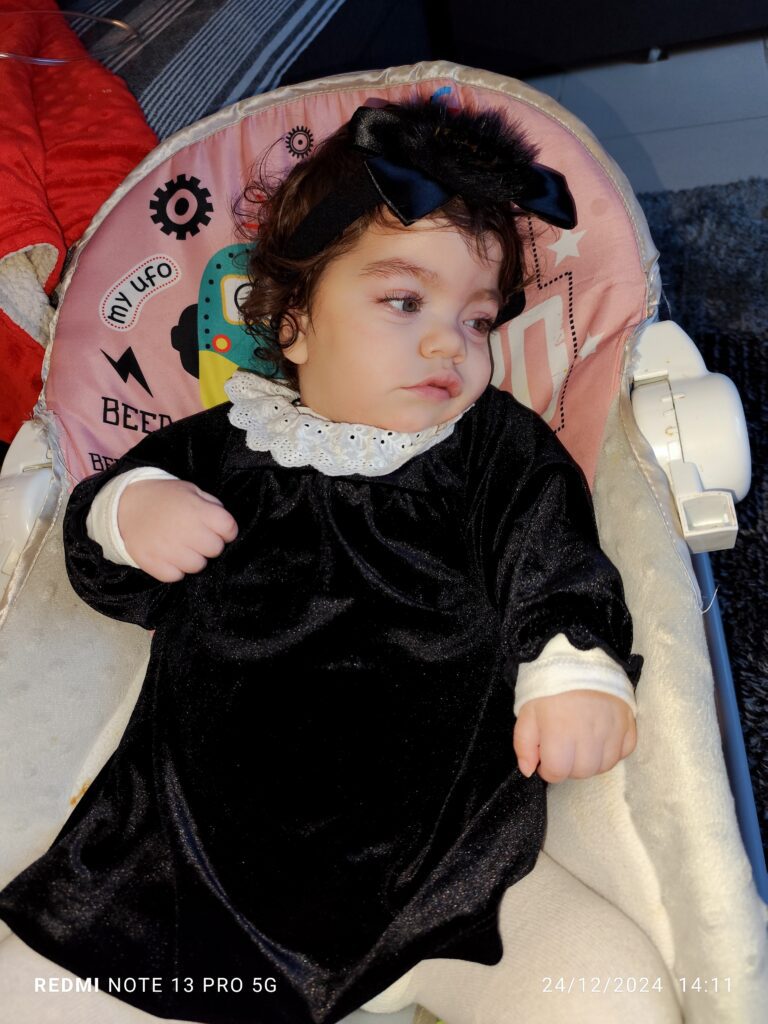
“My name is Allana Vitória, I am 12 months old and I’m from Lisbon, Portugal. I have GM1 type 1. I was diagnosed when I was just one month old, while I was in the hospital because I was born premature. My father thought my swollen eyes were strange and that something wasn’t right, as I already have two older siblings – my brother Kevim who is 10 years old and Lorrany who is 5 years old – and when they were born, they were more active than me.
The confirmation came quickly because my dad went to look at old medical reports of my maternal aunt who also passed away from a disease, which they didn’t know the name of and had little information about, as she passed away more than 20 years ago. But that night when my parents read my aunt’s report, which my grandmother had kept, it was shocking to read each word, as the symptoms were exactly the same as mine.
The next day, the neonatal doctor called a genetics doctor, and this doctor said it was unlikely she had this disease because the disease doesn’t pass from aunt to niece, but unfortunately, my parents, by great misfortune, have the same defective gene. But I remain active, I have a big family that gives me lots of affection, and I enjoy the kisses from my siblings and parents and some tickles and playtime.”

A Message From Christine about Iris’s birthday
Community Engagement
This month, as we prepare for Rare Disease Day, Iris celebrates her 17th birthday. Each birthday is a precious milestone that doctors once thought she might not reach. When she was diagnosed at age 5, we were told she had 5-10 years to live. Today, she continues to fight, smile, and inspire us every single day.
But this day and all our work at Cure GM1 isn’t only about Iris. It’s about every child with GM1 – the ones diagnosed and undiagnosed, the families searching for answers, and the researchers working to find treatments.
With a rare disease such as GM1, we face a challenge where the most interested parties in our cause are affected families. Fundraising when overwhelmed with complex medical care and a fatal disease can be truly difficult. Every contribution helps us so that one day families might find some relief from this truly difficult journey.
Follow Iris’s Journey

WORLD Symposium GM1 Poster Presentations
COMMUNITY & ENGAGEMENT
Christine Waggoner, Tayara Calina, and board members Vanessa Rangel Miller and Rafael Escandon are pleased to represent Cure GM1 at the WORLD meeting. We’re very proud to have prepared two posters and abstracts this year. A special thank you to our collaborators Dr. Caroline Hastings of UCSF and Picnic Health and to all those who have contributed to our data-sharing work!
We will release our posters in the next newsletter for those interested in reading the presentations and abstracts. We look forward to catching up with key opinion leaders, biotechs, and collaborators in person. Sadly, NIH presentations were canceled due to a recent government communications pause.
Below are GM1-related posters and presentations that are scheduled to be showcased at the WORLD meeting
Altered ganglioside pattern in the infantile form of GM1 gangliosidosis identified using reversed-phase chromatography combined with thin-layer chromatography Maroua Jakani
Effect of GC200, a novel orally available pharmacological chaperone (PC), on GM1- ganglioside reduction in the brains of hR201C Tg, Glb1KO/ mice as a promising preclinical candidate Sunhee Kang
*Genotypic Heterogeneity in GM1 Caroline Hastings
Penetrating the blood-brain barrier: Utilizing the PS gene editing system to encode a novel fusion β-galactosidase for the treatment of GM1 gangliosidosis Michael Pryzbilla
Clinical, neurological, and neuroradiological outcomes of intravenous AAV9 gene therapy in infantile GM1 gangliosidosis patients Albert Yang
*The GM1 Natural History Data Sharing Collaborative: A roadmap for accelerating rare disease research Christine Waggoner
Gangliosidoses: Understanding disease evolution in GM1 and GM2 Roberto Giugliani
Volumetric magnetic resonance imaging and diffusion tensor imaging metrics correlate with clinical outcomes following gene therapy in GM1 gangliosidosis patients Connor Lewis
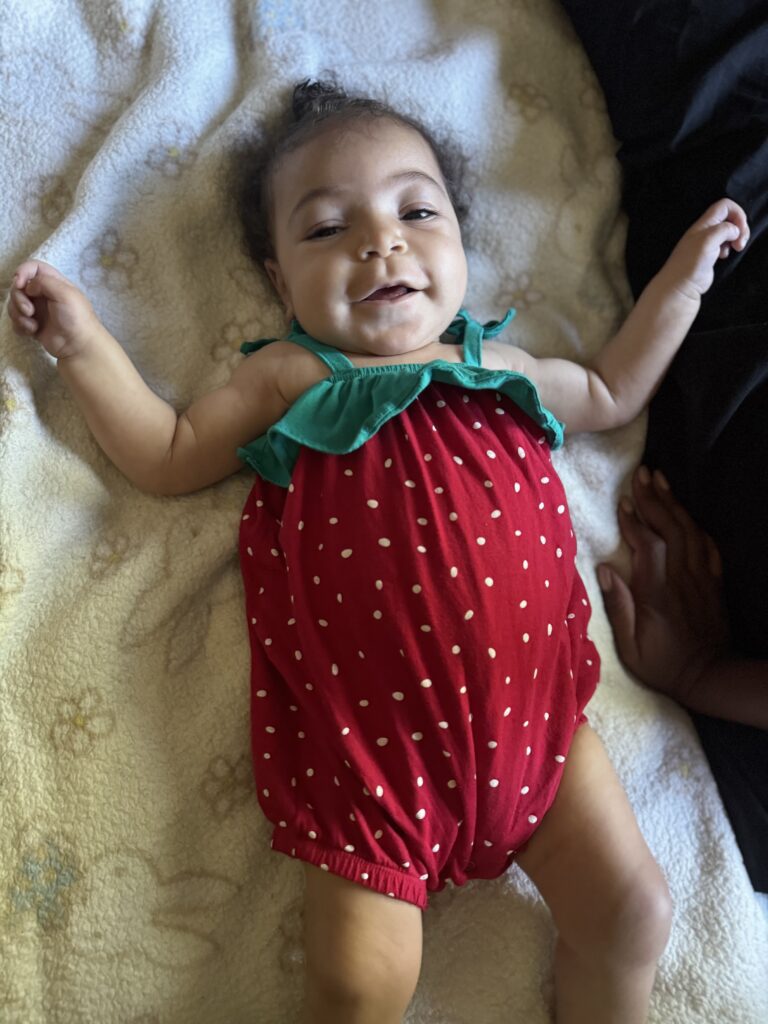
Milah’s story, by her mother
COMMUNITY ENGAGEMENT
At just six months old, Milah’s parents noticed she wasn’t meeting her developmental milestones. She lost her hearing and never had the chance to sit, crawl, or speak. When they finally received the heartbreaking diagnosis of GM1 gangliosidosis, their world was forever changed.
Despite the challenges, Milah is full of joy—she never cries and fills her family’s life with love. But GM1 has taken away so much, forcing them to reimagine every dream they had for their first child. Instead of planning for school and family trips, they are focused on cherishing every moment, creating memories, and finding ways to communicate with their daughter.
“No parent should have to hear that their child has a disease with no treatment or cure—that they won’t live long. We need to give a voice to the babies who can’t speak for themselves.”
GM1 is relentless, but Milah’s story is a reminder of why we fight for a cure. With more awareness, research, and support, we can bring hope to families like hers.
This Valentine’s Day, show some love while supporting Cure GM1. Check out the latest designs and new ways to give back at our GM1 Store!

Purchase Prints and Cards to Support Cure GM1 year-round with Minted.com!
For a limited time, your supporters can enjoy 20% off all Valentine’s Day orders plus FREE shipping using your unique code FUNDRAISECGM1. Hurry—this offer is available until January 28!
Rare Disease Innovation Hub Releases Strategic Agenda
ADVOCACY
Recently, the FDA’s Rare Disease Innovation Hub (the Hub) released its Strategic Agenda, which outlines the actions the Hub plans to undertake during its first year with extensive involvement from the rare disease community, while also addressing questions about the ultimate structure and programs of the Hub. The Strategic Agenda reflects extensive input from and partnership with the larger rare disease community, and identifies the following goals for the Hub:
Goal 1 – Further Advance Regulatory Science of Rare Disease Therapies
Goal 2 – Enhance and Strengthen Coordination and Alignment Between FDA’s Medical Product Centers, with Particular Focus on the Center for Biologics Evaluation and Research (CBER) and the Center for Drug Evaluation and Research (CDER)
Goal 3 – Create a Centralized Point of Contact for External Partners
It is the hope and expectation of FDA leadership and the Hub that this agenda will evolve as the Hub evolves. It is also the expectation that the rare disease community will remain heavily engaged in this evolution. Throughout the year, there will be multiple ways for the community to engage with and offer suggestions to the Hub, both for immediate use and for consideration for the Hub’s 2026 Strategic Agenda.

Save the Date! The Cure GM1 Conference 2025
ADVOCACY
Join us in person on August 8, 2025, in Irvine, CA!
Thinking about a summer Disneyland getaway? This is the perfect opportunity to combine a meaningful event with some family fun! Conference registration opens in spring!
Interested in volunteering or helping with the event? We’d love your support! Email info@curegm1.org to get involved. Stay tuned for more details!

Advocacy Letter to Trump Administration Led by the EveryLife Foundation
ADVOCACY
Cure GM1 was proud to join 209 partner patient advocacy organizations in outlining the collective patient community’s policy priorities in a letter to the Trump Administration.
The key policy areas highlighted for consideration included:
✔️ Preserving and enhancing therapeutic development incentives
✔️ Supporting predictable and consistent approaches to the evaluation of rare disease therapies
✔️ Modernizing the nation’s newborn screening program to ensure all babies can benefit from lifesaving health interventions as early as possible
✔️ Supporting policies that facilitate timely and affordable access to approved therapies
Together, we are advocating for policies that improve the lives of those affected by rare diseases.

GM1 Day is in May
ADVOCACY
You can make a difference this GM1 Day by helping us advocate for an official proclamation in your state! If you live in one of the eligible states, we need your voice to request recognition for GM1 Day as an official day of observance.
Proclamations can be requested every year, even if you’ve already received one! Interested in helping? Email us at info@curegm1.org.

Get Paid to Take Rare Disease Surveys
ADVOCACY
At Rare Patient Voice you can sign up to take paid surveys, phone interviews, online bulletin boards, focus groups, clinical trials, and more at a generous $120 per hour. For each person who signs up, Cure GM1 will receive $10. This research helps progress research into treatments and therapies for GM1. Sign up today!
Cure GM1 Completes Data Analysis Plan for xCures Pilot Study
BIOTECH NEWS
Cure GM1 has completed enrollment of the initial pilot study for our xCures real-world evidence pilot study. Throughout 2024, Cure GM1 has partnered with xCures to continue to advance a highly customized pilot data analysis plan. After an extremely thorough process of medical record collection, we are pleased to reach the milestone of detailed analysis for the first participants in the study. The learnings from this study will better inform interested parties of the potential of this approach and help enhance future work. The data obtained from this pilot will also be possible to contribute to our GM1 Data Sharing Initiative.

Cure GM1 Allocates $400,000 to Further Develop Enzyme Replacement Therapy
BIOTECH NEWS
Cure GM1 is dedicated to investing in research, seeding new projects, and to the development of multiple treatment modalities for GM1 gangliosidosis. In collaboration with our advisors and consultants, who have decades of experience in biotech and research, Cure GM1 has selected a contract research organization (CRO) to further the development of enzyme replacement therapy (ERT).

GEMMABio Appoints Dr. May Orfali as Chief Medical Officer
BIOTECH NEWS
“Dr. Orfali brings nearly three decades of experience in clinical development and medical affairs. She has expertise in multiple therapeutic areas, including oncology, infectious diseases, and rare and orphan diseases. She has led clinical development, clinical operations, biostatistics, medical affairs, patient affairs, and regulatory and strategy execution for several successful companies.”

Empowering Patients 2025: A Cell and Gene Therapies Summit
BIOTECH NEWS
“This inaugural virtual event March 12 and 13, 2025 (9 a.m. – 12:30 p.m. ET on both days), will provide timely education and engaging discussions on cell and gene therapies (CGTs). In alignment with ASGCT’s strategic plan, the goal is to empower advocates, patients, and caregivers who are vital in developing treatments. Attendees will hear from experts in the CGT field, as well as patient advocacy group leaders. There will be opportunities to ask questions and deepen your knowledge of the community’s CGT journey.”

Welcome to the GM1 Community, and Happy Birthday to Ezra!
Join us in welcoming Ezra, who just turned 2 years old. This little gentleman is full of love, light, and the sweetest smiles.
Ezra, you are part of a community that cares deeply and is working toward a brighter future for all children with GM1. We are so happy to have you and your family with us!