CURE GM1 CATALYST
Don’t miss all that is happening in our community and the broader rare disease community! There are many important opportunities to engage and to make a difference! Read on to learn more on continued progress and help build momentum.
Table of Contents:
COMMUNITY ENGAGEMENT
ADVOCACY
BIOTECH NEWS
FUNDRAISERS
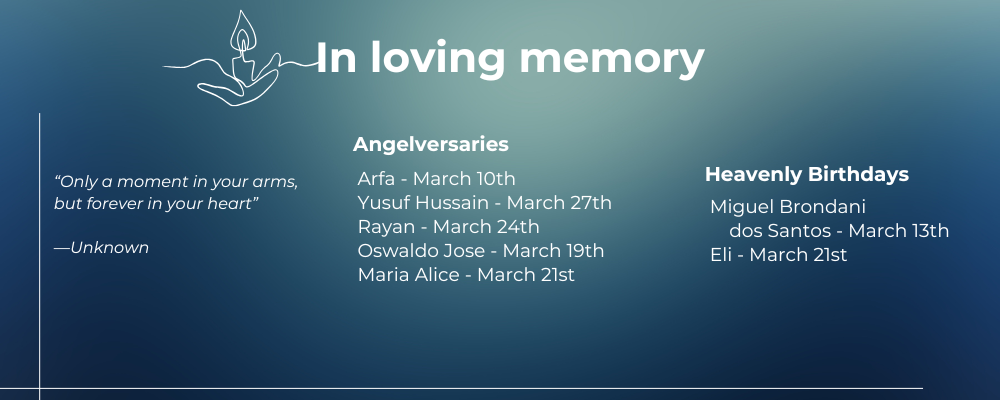
ANGELVERSARIES & BIRTHDAYS
Community Engagement

Rare Disease Day Photo Contest Winner!
Peyton is the winner of our 2025 Rare Disease Day photo contest winner. Join us in congratulating her, her big sister, and the rest of the family!
Missed the photo contest? Submit your story to be featured and to help spread awareness here.
Join Our State Proclamation Campaign!

Help us make history by getting May 23rd, 2025 officially recognized as GM1 Gangliosidosis Awareness Day in your state! We’ve prepared all the materials you need – proclamation requests, supporting documents, and step-by-step guidance. Many states require submissions months in advance, so we need to start acting now!
Your state’s proclamation could help future families get faster diagnoses and better support. We need to bring visibility to our cause, and Proclamations are a great way to achieve statewide recognition.
Ready to help? Email tayara@curegm1.org with “GM1 Proclamation – [Your State]” in the subject line. Together, we can amplify our voice and make a real difference for the GM1 community!
Show Your Support for gM1 Awareness – Check out the Mother’s Day Gear!
Featured Storyl

Gael’s Story
Meet sweet Gael, a bright-eyed little boy just over a year old. Diagnosed with Type 1 infantile GM1 on his first birthday, Gael and his family face this rare disease with strength and unwavering faith. Despite the challenges, his mother’s message reminds us that hope and unity can drive meaningful change in the rare disease community.
Join the Cure GM1 Foundation Board of Directors
Want to contribute in a truly meaningful manner on a regular basis? Cure GM1 is currently accepting applications for the board of directors which is a working board. We are specifically seeking applicants with professional skills to help support the organization. If you would like to see the job description and application, please mail info@curegm1.org

Thinking about a summer Disneyland getaway? This is the perfect opportunity to combine a meaningful event with some family fun!
Interested in volunteering or helping with the event? We’d love your support! Email info@curegm1.org to get involved. Stay tuned for more details!
We are prepping an exciting agenda for our August conference and already, we have many speakers and talks scheduled. Recently, two confirmed speakers have been in the news! Don’t miss out and save the date to join us in August!
Tippi MacKenzie

Tippi MacKenzie is a pediatric and fetal surgeon who is focused on developing better ways to diagnose and treat genetic diseases before birth. She leads a translational research lab examining the unique biology between the mother and her fetus, with the idea that pregnancy complications such as preterm labor arise from a breakdown in maternal-fetal tolerance. At present, the MacKenzie group is focused on preterm labor and fetal therapies including stem cell transplantation, enzyme replacement therapy, and gene therapy.
Terry Pirovolakis

Don’t miss this moving interview on The Kelly Clarkson Show, where Terry, a father from Canada with no medical background, shares his incredible journey of creating the world’s first gene therapy to save his son from an ultrarare genetic disease. It’s a story of hope, resilience, and the power of one family’s determination—watch it now!
In Loving Memory of Aidan and Theodore

This February, our GM1 community said goodbye to two beautiful angels – Aidan and Theodore.
Aidan was a vibrant child who walked, talked, climbed, and loved devouring turkey legs. During bedside stories, he would express interest with wide-open eyes, or show his disapproval if mom stopped before he was ready to be done.
Read Aidan’s story told by his mom

Theodore’s pure joy found expression in music, sensory experiences, and the simple pleasures of life, like kisses and cuddles, and hearing birdsong. His giggles and expressive movements were his way of sharing happiness with those around him.
Watch Theo’s story in his mother’s words
They taught us profound lessons about love, resilience, and the value of enjoying every moment. Their stories highlight the urgent need for greater support for rare disease research and treatment development.
We hold their families in our hearts as they navigate this difficult time. Aidan and Theodore’s legacies live on through the awareness they’ve raised and the lives they’ve touched. They remind us that every child’s life can create ripples of change that continue long after they’ve left us.
Advocacy
The Rare Pediatric Disease Priority Review Voucher (PRV)
The PRV program expired on December 20, 2024, meaning the FDA can no longer grant PRVs unless the drug was designated for a rare pediatric disease by that date and receives approval by September 30, 2026. The House passed the Give Kids a Chance Act of 2024 that would extend the designation deadline until September 30, 2029, but it was not included in the final government funding extension.
On February 12, 2025, Representatives McCaul (TX) and Dingell (MI) reintroduced the Give Kids a Chance Act, H.R. 1262, in a bipartisan effort. This Act includes a five-year reauthorization of the PRV Program and policies to increase access to pediatric cancer clinical trials and clarify the application of the Orphan Drug Act’s exclusivity provisions.
What can you do?
You can urge your members of Congress to reauthorize the program by passing the Give Kids a Chance Act of 2025 (H.R. 1262). Visit the Action Alert to contact your legislators!
Act For Ultrarare
We are proud to collaborate on advocacy for this new Act for Ultrarare.
The Act for Ultrarare initiative, organized by Ultragenyx, advocates for updated national policies to accelerate the development of treatments for ultrarare diseases—conditions affecting fewer than 2,000 individuals in the U.S. Recognizing that the original Orphan Drug Act of 1983 doesn’t adequately address the unique challenges of these diseases, the initiative proposes a framework that includes qualifying biomarkers as primary endpoints in clinical trials, accepting alternative study designs suitable for small patient populations, offering financial incentives like tax credits and extended marketing exclusivity, and ensuring broader access to genetic testing for early diagnosis. These measures aim to remove barriers in drug development and bring innovative therapies to those with ultrarare conditions.
This website is not available to view outside the U.S. due to legal considerations.
NIH and FDA Changes, Medicaid Funding Cuts, and the Department of Education Elimination: What Families Need to Know
The federal government proposed drastic changes to the National Institutes of Health (NIH) and Food and Drug Administration (FDA) that could significantly impact GM1 research. Recent attempts to cap NIH’s indirect funding at 15% (down from 30-70%) would remove billions from the research infrastructure supporting rare disease studies, potentially delaying or derailing projects close to clinical trials. Though federal judges have temporarily blocked these cuts, uncertainty remains. Meanwhile, extensive layoffs across scientific agencies have removed specialized expertise in fields critical to rare disease research, with hundreds of FDA, NIH, and CDC staff terminated (though some FDA medical device reviewers were later reinstated after industry pushback).
We are closely following the proposed $880 billion in Medicaid cuts, which would disproportionately impact vulnerable populations, including children with rare diseases who rely on this coverage for essential treatments and important services, forcing difficult choices between healthcare and other necessities for families already facing significant challenges. We also continue to monitor the proposal to eliminate the Department of Education which could impact IEPs and school support.
For our GM1 community, these disruptions and proposed changes threaten the steady progress we’ve made toward treatments and services provided to families.
We remain committed to our community and our critically important work.
View the Action Alerts to Contact Congress Here
Advocacy and the critical work at Cure GM1 must continue in the face of these concerning changes to our public health infrastructure. Please consider ongoing support or a donation today.
Advocacy Events and Opportunities
The Orphan Disease Center (ODC) and Critical Path for Lysosomal Diseases (CPLD) will be hosting a series of webinars focused on data sharing over the next four months. Over the course of the series, attendees will learn about current best practices for sharing data, specifically within the lysosomal storage disease (LSD) community. The goal of the webinar series is to provide patient advocates, sponsors, and study participants with a toolkit for sharing data and to empower the formation of data sharing collaborative projects.
25 Million Wishes is an organized movement by Rare Village to shed light on the millions of kids in North America who live with rare diseases. The website serves as an opportunity for parents and caregivers to upload a photo of their rare kiddo with some basic information.
The photos and bios will be displayed in a gallery on the website, and each kid will have their own profile page. Ultimately, these images will all be printed off and displayed on Capitol Hill as a powerful message that superficial funding and programmatic decisions have such very deep impacts on the lives of very real Americans.
Now, more than ever, it is important to make sure the voices of the most vulnerable are heard in the halls and offices of our policy makers.
We need all the participation we can get! Together, we can collectively make sure all 25 million rare kiddos live to wish as many wishes as they can.
Support Your Family Wishes- Courageous Parents Network’s Webinar
If you’re navigating tough medical decisions for a loved one, this free webinar by Courageous Parents Network offers expert guidance on how to communicate and honor your family’s wishes. Gain practical strategies and emotional support to feel more confident in advocating for your family’s needs—don’t miss this valuable discussion!
Cure GM1 Presented Two Posters and Abstracts at the WORLD Symposia Meeting in San Diego

Cure GM1 is proud to present two projects that have been multi-year endeavors. We presented the analysis of genotypes, signs, and systems from data collected on the AllStripes platform which was acquired by Picnic Health for a cohort of 26 participants.
In addition, we presented a poster on the formation of a GM1 data sharing collaborative group. Due to the rarity of GM1 and also the large number of studies, it is imperative to continue to encourage aggregation and sharing of data to deepen the understanding of GM1 and to reduce the need for new natural history studies or data which is siloed.
Biotech News
Dr. James M. Wilson honored with the prestigious 2025 Roscoe O. Brady Award

On Tuesday, February 4, at WORLDSymposium 2025, Dr. James M. Wilson was honored with the prestigious 2025 Roscoe O. Brady Award. Renowned as a pioneer in the field of gene therapy, Professor Wilson created the first and largest academic-based program in gene therapy.
We are so grateful to Dr. Wilson for prioritizing GM1 gangliosidosis and making it the lead program at GEMMABio!

GC Biopharma
We are excited to share that GC Biopharma presented development updates on its LSD (lysosomal storage disorder) pipelines at the WORLD Symposium 2025, held from Feb. 3rd to 7th, 2025, in San Diego, USA.
GC Biopharma’s poster presentations on the non-clinical study results of “GC2025A”, its oral chaperone therapy for GM1 gangliosidosis (GM1), and “GC1130A”, for Sanfilippo syndrome type A (MPS IIIA).
At its GC2025A poster presentation, GC Biopharma unveiled promising non-clinical trial data for its GM1 candidate drug. The results demonstrated that, when administered orally to GM1 animal models over a 7-day period, the drug led to a remarkable reduction of more than 70% in GM1 levels within the brain.

Azafaros
The Cure GM1 team attended multiple meetings and presentations by Azafaros at the WORLD meeting. The planning for the global Phase 3 clinical trial is underway. Additional updates regarding the future Phase 3 study will be provided when new updates are publicly available.

Empowering Patients 2025: A Cell and Gene Therapies Summit
“This inaugural virtual event March 12 and 13, 2025 (9 a.m. – 12:30 p.m. ET on both days), will provide timely education and engaging discussions on cell and gene therapies (CGTs). In alignment with ASGCT’s strategic plan, the goal is to empower advocates, patients, and caregivers who are vital in developing treatments. Attendees will hear from experts in the CGT field, as well as patient advocacy group leaders. There will be opportunities to ask questions and deepen your knowledge of the community’s CGT journey.”
Fundraisers
Mother’s Day Merch!
Make a fundraiser this year!
With all the recent developments with respect to cuts to biomedical research and the public health infrastructure, the work of Cure GM1 is more important than ever. We cannot sit idly and lose ground. Your support matters most in these challenging times.
Our work with respect to our new enzyme replacement therapy project is moving forward. Your support can help move this program forward to help contribute to an arsenal of tools to treat GM1.
Please join us by making a commitment to having a fundraiser this year.

Ways to Give – Your Support Matters
RaiseRight | iGive | Charity Miles | Facebook Fundraisers | Donate |
Set up a Recurring Donation
Visit our Take Action page for more ways to support our community.
Cure GM1 Foundation | PO Box 6890 | Albany, CA 94706 US







