October News!
The Cure GM1 Catalyst
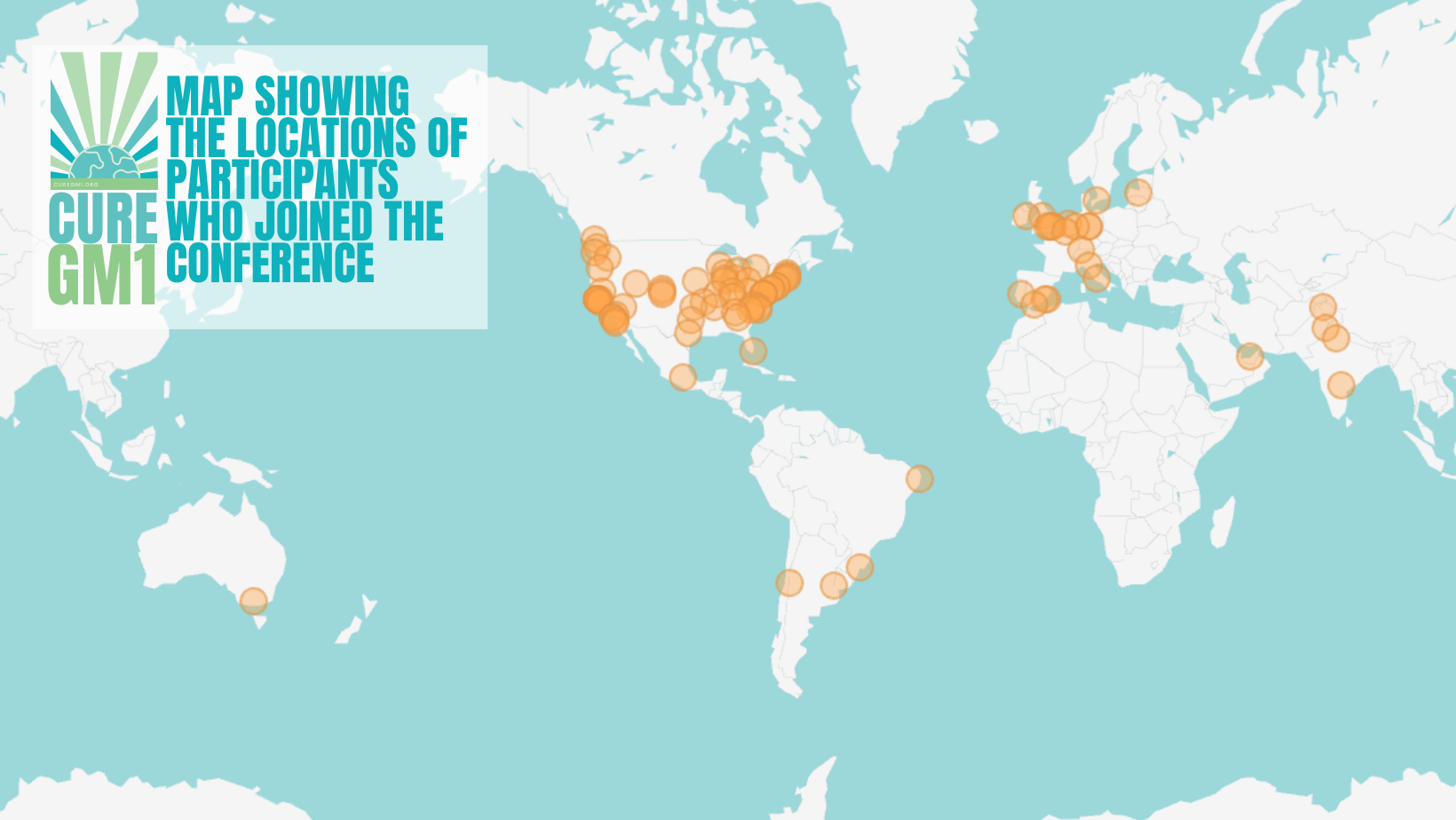
Welcome to our latest newsletter, where we celebrate our resilient community and the recent strides made in the fight against GM1 gangliosidosis. From the highlights of the 2024 Cure GM1 conference, which brought together participants from 21 countries. With promising updates and recent news, we continue to strengthen our global network and to foster hope. Together, we are making a difference and driving change for GM1 families worldwide. Let’s keep this momentum going!
Community Engagement
Fundraising
Biotech News
Angelversary | Birthdays
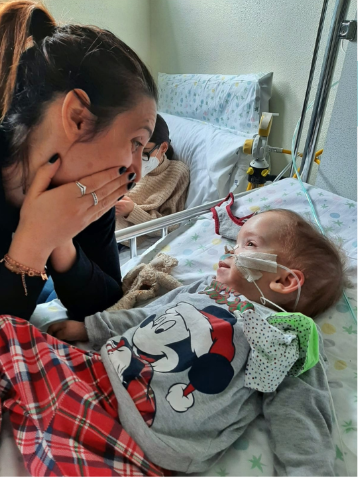
Highlights from the 2024 Cure GM1 Conference
COMMUNITY & ENGAGEMENT
Thank you to everyone who joined us at this year’s Cure GM1 Conference! With participants from 21 countries, this event was a powerful reminder of our community’s global reach and shared hope!


Conference Key Milestones & Updates
This was the first conference ever where there was a mention of a future Biologics License Application (BLA). While the future is still unknown, it was a very hopeful meeting. The Azafaros trial is planned to begin Phase 3 in 2025 and Gemma Biotherapeutics is launching and building a plan for their next steps towards further advancing their AAV program gene therapy for GM1, having acquired the license to carry forth work originally conducted by Passage Bio. Furthermore, NIH made two presentations, one regarding their ongoing intravenous AAV9 gene therapy and another presentation on their recent publication of 10 years of Type 2 natural history data, a huge achievement. Each year, this unique event continues to evolve. We are proud to participate in this evolution with new initiatives and progress.

Conference Recordings & Feedback
Registered attendees only have received recordings via email, available for one month. After that, select segments may be shared on our YouTube channel. Please complete our Conference Survey to share your thoughts.
We encourage you to reach out if you would like to learn more about the All Needs Planning group who made a great presentation regarding financial planning for special needs families.
If you appreciated the conference, consider donating to support our work and future events. Your generosity makes a difference!
What Attendees Are Saying about 2024 Conference
“It was such an honor to take part in today’s conference. Thank you so much to the Cure GM1 Foundation for organizing such a wonderful day.”
-Attendee
“Thank you to the Cure GM1 Foundation for organizing this meeting, and to all the speakers who have made it possible for us to learn about the current progress in the fight against GM1, both in clinical trials and in patient or caregiver care.”
-Attendee

Diego’s Day: A Heartfelt Walk to Shine a Light on GM1 Gangliosidosis
COMMUNITY & ENGAGEMENT
At the recent fundraiser walk held at the Imola Autodrome, we were reminded of the profound impact of Diego, a cherished member of our GM1 community. His story, shared by his family during the event, highlights the dual nature of their journey—intense sadness paired with profound joy.
The event successfully raised funds, contributing to ongoing research and support initiatives for GM1 gangliosidosis.
Today, as we remember Diego, we also acknowledge the continuing struggle against GM1. This disease has not stopped; families worldwide are still fighting. It’s a poignant reminder of our mission at Cure GM1 Foundation—to push forward in the face of adversity, honoring those we have lost by continuing the fight for those still battling this relentless disease. This walk was for Diego, for every child who fights GM1, and for the futures we aim to change through our collective efforts. Read Diego’s Story>
Insights and Resources for GM1 Community
COMMUNITY & ENGAGEMENT
Christine Waggoner, our founder and president recently participated in the Global Genes conference to gather valuable insights and resources for the Cure GM1 Foundation from the broader rare disease community. Discussions at the event spanned crucial topics from legislative advocacy to collaborative opportunities, vital for our continued work to advance possible treatments for GM1 gangliosidosis. For those in our community seeking to deepen their understanding or access supportive tools, the Global Genes Community Guides provide a wealth of information, accessible.
Resources for the Rare Individual>

Celebrating Imogen’s Creative Spirit
COMMUNITY & ENGAGEMENT
We are excited to celebrate Imogen, who played the starring role in her film “Queen of the Fairies.” Her movie went on to win the LauraLynn Oscar for Best Entertainment Movie, a well-deserved recognition of her talent and creativity. Imogen’s incredible creativity and determination shine through in her film, showcasing the power of imagination and the joy it can bring to those around her. Her achievement is a testament to the strength and resilience of children in our GM1 community. Read more>
Please reach out to the Cure GM1 with your stories and recommendations so we can continue to uplift and inspire each other.
FDA Rare Disease Innovation Hub to Enhance and Advance Outcomes for Patient
COMMUNITY & ENGAGEMENT
Stay informed about the FDA’s latest efforts to enhance outcomes for patients with rare diseases. The FDA Rare Disease Innovation Hub is dedicated to advancing research and therapies. Learn more about their initiatives and how they impact our community.
Attend the upcoming FDA Rare Disease Innovation Hub Meeting on October 16th! It’s a fantastic opportunity to learn about the latest advancements in rare disease therapies. You can attend virtually, so don’t miss out on this chance to be part of the discussion and advancement in rare disease treatment.
FDA Rare Disease Innovation Hub>

Update on the Rare Pediatric Voucher Program Status
COMMUNITY & ENGAGEMENT
The Rare Pediatric Voucher program has been extended until the end of the year, but we need U.S. citizens to contact their senators to push for its renewal before year-end. While it has passed the House, it has yet to pass the Senate.
Read More>
xCures Collaboration Now Featured on ClinicalTrials.gov: Explore Our Real-World Data Study on GM1
COMMUNITY & ENGAGEMENT
Stay informed about our latest collaboration with xCures, now officially listed on ClinicalTrials.gov. This pivotal partnership focuses on gathering real-world data to enhance our understanding of GM1’s natural history. Learn more and follow our progress. General xCures project.

Thank you Fiona’s Kindness Project
COMMUNITY & ENGAGEMENT
Special Thank you to Fiona’s Kindness Project for their yummy egg roll fundraiser! We are so thankful for the support in honor of beautiful Fiona who suffered from infantile GM1 and for her family’s dedication to honoring her memory and our cause.

Last Chance for Fall Bulb Orders!
FUNDRAISING
Don’t miss out on your opportunity to order fall bulbs and support a great cause. Place your order now before it’s too late. Order Here>

Plan Your Year-End Giving!
FUNDRAISING
As the year comes to a close, it’s the perfect time to start planning your charitable contributions. Mark your calendars—Giving Tuesday will be on December 3rd in 2024. Let’s make a difference together!

Fall See’s Candies
FUNDRAISING
Treat yourself or a loved one to delicious See’s Candies chocolates this fall, and a portion of your purchase will support GM1 research. Order now and make your sweet treats count for a great cause!
Order Here>

Support Cure GM1 year-round with Minted.com!
fundraising
Use the code FUNDRAISECGM1 at checkout to receive a special discount on your purchases. Plus, 15% of your purchase will be donated to charity. It’s a simple way to give back while shopping for beautiful, high-quality products. Every purchase helps make a difference for those affected by GM1 gangliosidosis.
Visit Minted.Com to support Cure GM1 >

Two Approvals in Niemann Pick C Offer Hope to The Rare Disease Community
biotech news
It’s been a very eventful time for Niemann Pick C (NPC), a neurodegenerative lysosomal disease like GM1. These two approvals offer hope to rare diseases and to lysosomal diseases such as GM1 gangliosidosis with a high unmet need for treatment.
PRESS RELEASES:
IntraBio Announces U.S. FDA Approval of AQNEURSA for the Treatment of Niemann-Pick Disease Type
Zevra Therapeutics’ MIPLYFFA™ (arimoclomol) Receives U.S. FDA Approval as Treatment for Niemann-Pick Disease Type C
Congratulations to Zevra Therapeutics and to IntraBio Inc / IntraBio Ltd. on these milestones and achievements.
IntraBio also conducted a clinical trial for GM2 gangliosidosis. However, at present, AQNEURSA is approved for NPC only. Please always consult your physicians regarding medications and care.
Positive Niemann-Pick disease type C (NPC) and GM2 gangliosidosis data from nizubaglustat Phase 2 RAINBOW study conducted by Azafaros presented at major metabolic disease conference
biotech news
“Leiden, The Netherlands, 10 September 2024 – Azafaros has announced that data from the ongoing double-blind, placebo-controlled Phase 2 RAINBOW study investigating its lead asset, nizubaglustat in patients with Niemann-Pick disease type C (NPC) or GM2 gangliosidosis, were presented at the Society for the Study of Inborn Errors of Metabolism (SSIEM) annual symposium in Porto, Portugal. The results of part one of the study, designed to determine the safety, pharmacodynamics, and pharmacokinetics of the Company’s lead asset nizubaglustat, demonstrated the compound had a positive safety profile and was well-tolerated in the 13 participants in the study.” The RAINBOW study did not include GM1 gangliosidosis, but GM1 will be part of the upcoming Phase 3 study and the completion of Phase 2 means we are one step closer to Phase 3.
Read More>

JCR Pharmaceuticals’ Data Presentations at SSIEM Annual Symposium 2024 Highlight Investigational Treatments for Lysosomal Storage Disorders
biotech news
“GM1 Gangliosidosis Poster poster presentation was selected as one of the highest-ranked posters. It highlights preclinical data from an adeno-associated virus (AAV) gene therapy in a mice model of GM1 gangliosidosis: Gene therapy for GM1 gangliosidosis mediated by AAV vector carrying BBB-penetrable enzyme (PO-211)
Presenter: Saki Matsushima, Ph.D. (Division of Gene Therapy, Research Center for Medical Sciences, The Jikei University School of Medicine, Tokyo, Japan)”
Read More>

IN LOVING MEMORY
We extend our love and support to Harrison Blake’s family and all who were touched by his life. Harrison passed away peacefully, on Tuesday, September 17, 2024, at the age of 4 years. His strength and spirit left a lasting impact on the Cure GM1 community. We honor his memory as we continue our mission to find a cure.